Abstract
Background: Sickle cell disease (SCD) is a life-threatening, hereditary hemoglobin (Hb) disorder characterized by chronic hemolytic anemia, pain, end-organ damage, and poor quality of life. The key pathology is red blood cell (RBC) sickling due to polymerization of deoxygenated sickle Hb (HbS), which can be exacerbated by increased levels of the glycolytic metabolite 2,3-diphosphoglycerate (2,3-DPG), and decreased ATP. Sickled RBCs are rigid, not deformable, and fragile, resulting in vaso-occlusion triggering pain and chronic hemolysis. SCD treatment options are limited, with an unmet need for safe and effective therapies to improve anemia and reduce pain. Mitapivat is an investigational, first-in-class, oral, small-molecule allosteric activator of the RBC-specific form of pyruvate kinase (PKR), a key enzyme in glycolysis. Activation of wild-type PKR decreases 2,3-DPG and increases ATP, which may reduce HbS polymerization, RBC sickling, and hemolysis in SCD. Data from the Phase 1 National Institutes of Health multiple ascending dose study of up to 100 mg mitapivat twice daily (BID) in SCD (NCT04000165) showed that mitapivat was safe and tolerable, and was associated with a dose-dependent increase in ATP and decrease in 2,3-DPG, in addition to improvements in anemia and hemolytic markers. Based on these results, a Phase 2/3 study investigating the safety and efficacy of mitapivat in patients (pts) with SCD is planned.
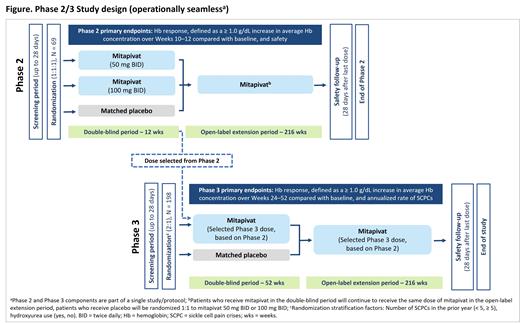
Methods: This Phase 2/3, double-blind, randomized, placebo-controlled, multicenter study aims to evaluate the efficacy and safety of mitapivat in pts with SCD. Eligible: pts ≥ 16 years of age with documented SCD (HbSS, HbSC, HbSβ 0/HbSβ + thalassemia, other SCD variants), 2-10 sickle cell pain crises (SCPCs; acute pain needing medical contact, acute chest syndrome, priapism, hepatic or splenic sequestration) in the prior 12 months, and an Hb level of 5.5-10.5 g/dL. If taking hydroxyurea (HU), the dose must be stable for ≥ 90 days before starting study drug. Not eligible: pts receiving regularly scheduled blood transfusions, with severe kidney disease or hepatobiliary disorders, currently receiving treatment with SCD therapies (excluding HU) or who have received gene therapy, bone marrow or stem cell transplantation.
In the double-blind Phase 2 part of the study, 69 pts will be randomized (1:1:1) to receive 50 mg mitapivat, 100 mg mitapivat, or placebo BID for 12 weeks. The primary objective of the Phase 2 part of the study is to determine the recommended Phase 3 dose of mitapivat by evaluating anemia and safety vs placebo via the following endpoints: Hb response, defined as a ≥ 1.0 g/dL increase in average Hb concentration over Weeks 10-12 compared with baseline; and type, severity, and relationship of adverse events (AEs) and serious AEs (SAEs).
In the double-blind Phase 3 part of the study, 198 pts who did not participate in the Phase 2 study will be randomized (2:1) to receive the selected Phase 3 dose of mitapivat or placebo, BID, for 52 weeks, stratified by the number of SCPCs in the prior year (< 5, ≥ 5) and by HU use. The primary objectives of the Phase 3 study are to determine the effect of mitapivat vs placebo on anemia in pts with SCD, measured by Hb response, defined as a ≥ 1.0 g/dL increase in average Hb concentration over Weeks 24-52 compared with baseline, and to determine the effect of mitapivat vs placebo on SCPC, measured by annualized rate of SCPCs. Key secondary endpoints include change over Weeks 24-52 compared with baseline in the following: average Hb concentration, indirect bilirubin, percent reticulocyte, and Patient-Reported Outcomes Measurement Information System ® (PROMIS) fatigue 13a Short Form scores; annualized frequency of hospitalizations for SCPC. Other secondary objectives include evaluation of effect on additional markers of hemolysis and erythropoiesis, additional clinical efficacy measures related to SCPC, additional patient-reported measures of fatigue and pain, physical activity, safety, and pharmacokinetics/pharmacodynamics of mitapivat.
Pts who complete the double-blind period of either the Phase 2 or Phase 3 part of the study will be eligible to receive mitapivat for an additional 216 weeks in the open-label extension period.
Results: Not yet available
Conclusion: This Phase 2/3 study will investigate the efficacy and safety of the pyruvate kinase activator mitapivat in pts ≥ 16 years of age with SCD and enrollment will begin in 2021.
Howard: Resonance Health: Honoraria; Novartis: Consultancy, Honoraria; Bluebird Bio: Research Funding; Forma Therapeutics: Consultancy; Agios Pharmaceuticals: Consultancy; Novo Nordisk: Consultancy; Global Blood Therapeutics: Consultancy; Imara: Consultancy, Honoraria. Kuo: Bluebird Bio: Consultancy; Apellis: Consultancy; Agios: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene: Consultancy; Pfizer: Consultancy, Research Funding; Alexion: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Bioverativ: Membership on an entity's Board of Directors or advisory committees. Oluyadi: Agios Pharmaceuticals: Current Employment, Current holder of stock options in a privately-held company. Shao: Agios Pharmaceuticals: Current Employment, Current holder of stock options in a privately-held company. Morris: Agios Pharmaceuticals: Current Employment, Current holder of stock options in a privately-held company. Zaidi: Agios Pharmaceuticals: Current Employment. Van Beers: RR Mechatronics: Research Funding; Novartis: Research Funding; Agios Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal